Diabetes & Hypoglycemia: Behind the Scenes
Introduction
Hypoglycemia is clinically defined as anytime plasma blood glucose falls below 70mg/dL. Usual symptoms revolve central nervous system dysfunction as a result of the brain starving of glucose. Glucose is necessary for all bodily functions, and the brain itself uses over half of all available glucose in the body to perform its usual tasks. While most common in diabetic type 1 patients, it is actually possible for any patient to experience hypoglycemia under certain conditions.
In this article, we will explore the clinical definitions of hypoglycemia, standard prehospital and in-hospital treatment for it, the pathophysiology behind it and how cells metabolize glucose, and a basic overview of the condition generally.
What is hypoglycemia?
As stated above, hypoglycemia is clinically defined as any time plasma blood glucose levels fall below 70mg/dL - that is, the body has insufficient levels of circulating glucose throughout the body to feed natural metabolic processes such as muscular movements and consciousness.
Hypoglycemia is most common with diabetes type 1 patients, but a variety of factors such as alcohol binging, insulin dosing, and pancreatitis can prompt hypoglycemia in an otherwise healthy patient.
What’s glucose? What’s glycogen? What’s insulin?
Glucose is the fuel of the brain and body. It is most commonly absorbed via the breakdown of complex carbohydrates in food such as galactose (in milk) and fructose (in starches). As the body breaks down food within the digestive system, enzymes from both the liver and the pancreas are used to break down these complex carbohydrates into glucose (Hantzidiamantis, 2022). Glucose is the simplest form of carbohydrates and is used in every organic metabolic process (Hantzidiamantis, 2022).
Glucose is then broken down through the krebs and citric acid cycles to form ATP, or adenosine triphosphate. Adenosine triphosphate is essentially raw energy on a chemical level. Whenever the body takes an action such as a muscular movement, nervous impulse, or processing sensory information in the brain, the body uses up ATP to do so.
The brain uses over half of all glucose circulating within the body on average. During times of increased stress or increased mental load (imagine you’re taking a test!), the brain actually increases its use beyond half.
There is a storage form of glucose known as glycogen. The existence of glycogen is what allows us to keep prolonged periods of times between meals without always developing hypoglycemia. Glycogen is a compact form of glucose that is used for storage. It is primarily found within the skeletal muscles and the liver, with the vast majority in the liver. During times of stress or increased metabolic demand, the body begins to use up the glycogen stores to meet the body’s needs.
Glycogen is actually what the hormone/medication glucagon works on. By providing glucagon in the field, EMS providers are able to prompt a hypoglycemic patient to begin to use the glycogen stores remaining in the liver.
Insulin is the main hormone used for the transport of glucose from the plasma/bloodstream into the cells themselves. Insulin is normally excreted by cells called the Islets of Langerhaans in the pancreas in healthy individuals. In diabetic patients, insulin is often injected from exogenous sources as a means to regulate blood glucose levels.
Insulin does not lower blood sugar alone - it is merely a transporter for glucose. Insulin is only lowering blood plasma glucose levels by allowing for the transfer of glucose into the cells themselves - thus depleting the blood plasma levels. Insulin allows for the actual binding of ATP to EMS Aware is actually working on an article fully exploring insulin’s mechanism of action and its various types, so stay tuned for more.
Clinical Manifestations of Hypoglycemia
Clinical manifestations of hypoglycemia are in part because of two factors - central nervous system (CNS) dysfunction and catecholamine surge (fight or flight). The catecholamine response is typically dominant in the beginning stages of hypoglycemia before significant CNS dysfunction occurs.
Catecholamines
Hypoglycemia is a state of significant metabolic stress for the body - after all, the body is trying to stay alive, and glucose is necessary for that. As a means to compensate for the initial stages of hypoglycemia, the body becomes sympathetic nervous system-dominant and prompts the release of sympathomimetic hormones called catecholamines.
Catecholamines are the basic fight or flight hormones primarily excreted from the suprarenal or adrenal glands above the kidneys. During times of stress, the body releases hormones such as epinephrine, norepinephrine, and glucagon. The two major hormones involved in responding to hypoglycemia are epinephrine and glucagon.
Epinephrine is the main hormone involved in this fight or flight. Think of what the body has to do during times of hypoglycemia - run to fight and kill food sources or find other humans to get help. Epinephrine helps accomplish that. It has an effect on almost all of the beta-adrenergic receptors, but we will highlight the major ones below.
Beta 1 Receptors: Beta 1 receptors are primarily responsible for a stimulating effect on the heart.
Stimulus of the b1 receptors of the heart prompts chronotropic, inotropic, and dromotropic effects (Libretexts, 2023). In other words, a patient will experience tachycardia, increased cardiac contractility (stronger contractions), and increasing impulse conduction.
This increase in heart rate and the strength of contractions allows the body to increase cardiac output as a means to maintain metabolism and end-organ perfusion.
Beta 2 Receptors: Beta2 receptors are primarily responsible for bronchodilation of the lungs.
Stimulus of the b2 receptors prompts bronchodilation (the opening of the bronchioles) in order to allow for increased airflow and better oxygenation. It also prompts a spike in respiratory drive and thus tachypnea as a means to increase oxygenation.
Beta 2 receptors also prompt glycogenolysis - the breakdown of glycogen - as a means to attempt to raise blood glucose levels (Libretexts, 2023). This helps the body mediate hypoglycemia and assists with the mechanism of action of glucagon.
Alpha 1 Receptors: Alpha 1 receptors are primarily responsible for peripheral vasoconstriction.
This allows the body to shunt and divert blood to the core, where the major organs are. This ensures that the body continues to perfuse organs such as the heart, lungs, and kidneys during times of shock (with hypoglycemia can develop into!)
Alpha 1 receptors will also cause vasoconstriction in the brain - potentially worsening AMS due to decreased perfusion (Libretexts, 2023).
Alpha 2 Receptors: Alpha 2 receptors are primarily responsible for supplementary effects to the above receptors.
The main roles that a2 receptors play in hypoglycemia is the stimulus of glycogen release and glycogenolysis and the inhibition of insulin release from the pancreas (if any is available).
This helps increase the blood glucose level while also preventing further drops from excess or inappropriate insulin release. The body already doesn’t have enough glucose in the plasma - we don’t want insulin shifting all of it out of the plasma and into the cells for its supply to be exhausted entirely.
Not all receptors are being discussed in full in this article.
At the same time, glucagon is being released by the alpha cells of the Islets of Langerhaans within the pancreas. Glucagon is responsible for stimulating glycogenolysis and the use of glycogen stores within the liver. This allows the body to compensate for hypoglycemia by raising blood glucose levels.
It is important to note that glucagon can only work when the liver has adequate glycogen stores to utilize. Many patients with severe, progressed hypoglycemia have already begun to use up or have exhausted their glycogen stores on their own. Factors such as alcohol intoxication preventing the actual creation of glycogen also play a role and can be a reason for inadequate glycogen stores in an otherwise healthy patient.
Central Nervous System
The brain needs two things - glucose and oxygen - to function properly. If one of them is missing, patients will begin to experience symptoms of CNS dysfunction. This includes both mental status changes and neurological deficits that may even mimic a stroke or other significant CNS event.
The central nervous system functions through two main “types” of nerves - the afferent and the efferent. Afferent nerves refer to nerves that receive sensory input at its source - such as how a mechanoreceptor senses touch on a part of the skin - and then send the information up to the central nervous system for processing. Efferent nerves are nerves that actually carry out an effect; that is, they the nerves that send information or directions back down to carry out a response to stimuli.
For instance, a nociceptor (pain sense receptor) in the hand may sense a knife cutting into a person’s hand during a fight. This signals the nociceptors in the hand to transmit a pain signal up to the brain via afferent nerves, where it is then processed. In response, the brain signals back down via efferent nerves and causes a contraction of the hand away from the danger source. The actual mechanism is too complex for the purposes of this article, but this is a working simplification.
Image sourced from Wikipedia.
During times of hypoglycemia, both the afferent and efferent nerves slowly become dysfunctional. As glucose levels drop, the brain is starved more and more of the necessary fuel it needs to process stimuli and respond to it.
Usual CNS symptoms associated with hypoglycemia include:
Altered speech patterns, including slurring
This is because of poor sensation in the face coupled with reduced fine motor control of the vocal cords, facial muscles, and lips.
Patients may also have difficulty actually formulating speech because of the associated CNS dysfunction.
Altered bodily sensation
Remember - the afferent nerve signals aren’t sending stimulus upwards to the brain properly. As a result, patients may have a decreased sense of pain, heat, or touch throughout the body.
Altered vision
The actual processing of vision after information is passed along by the optic nerve occurs in the cerebral cortex of the brain. The brain is able to process stimulus less accurately if starved of glucose.
Research shows that rods - the parts of the eye primarily responsible for vision under lower light conditions - are affected by hypoglycemia moreso than cones (Khan et al., 2011). As a result, patients experiencing hypgolycemia at night are at increased risk of injury due to sensory perception deficits.
CNS dysfunction also causes diplopia as it progresses.
Altered musculoskeletal function
As the brain fails to respond to stimulus, it cannot effectively send out efferent signals for the body to take actions. The signals that it is able to send out is often poorly coordinated
As the brain continues to be starved of glucose and blood plasma glucose levels drop, CNS dysfunction will worsen accordingly. Patients with extreme levels of hypoglycemia are prone to diabetic seizures. As insulin is released in an attempt to facilitate the transfer of glucose into the cells and thus continue normal metabolism, it prompts a shift in the balance of electrolytes from the extracellular to the intracellular space. In the brain, this can cause significant edema. This increase in capillary permeability and shifting of electrolytes can prompt an influx of electrolytes and thus water retention in the brain, prompting cerebral edema. With time, this can push against the walls of the cranial vault and even herniate in severe cases. Again, this is not a common finding. Most hypoglycemic seizures are the result of severe glucose-deprivation resulting in ectopic or erratic neuron firings in the temporal lobe of the brain (Dudley et al., 2022).
Finally, the brain can enter a diabetic coma as hypoglycemia progresses to its worst stages. This typically occurs with BGLs under 49 mg/dL (Cryer, 2007). As the brain is starved of glucose, it will shut down the least vital functions first before moving on to the lower levels of the brain, such as the brainstem. With time, patients will experience altered respiratory patterns and heart rate control as a result of severe hypoglycemia as the pons and medulla oblongata cease functioning.
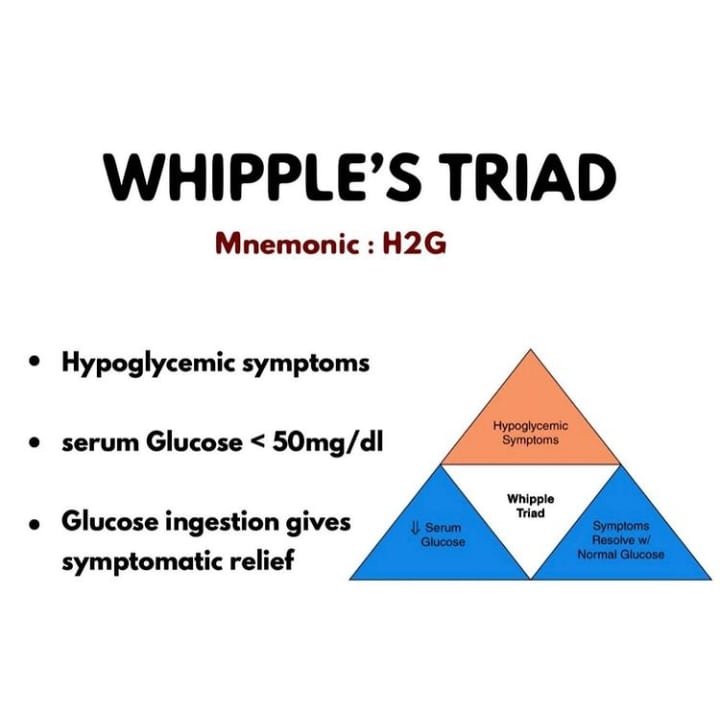
Patients experiencing hypoglycemia can be assessed using the Whipple’s triad. If a patient has hypoglycemic values along with associated clinical manifestations (AMS, sympathomimetic symptoms) that resolve with administration of dextrose and correction of hypoglycemia, it is likely a hypoglycemia issue. Whipple’s triad is often used for the diagnosis of insulinomas that cause excessive, inappropriate levels of insulin excretion from the pancreas.
Special Cases - Hypoglycemia
Sepsis
During times of metabolic stress - such as an infection - the body begins to use excess glucose compared to baseline as a means to fight off the infection. The body releases stress hormones such as epinephrine, glucagon, and cortisol - all of which have the effect of increasing glucose levels to meet demands. Glucose is used in things like WBC production, release, and transport.
People that are developing infections often have decreased oral intake and activity levels as a result of their sickness. However, a diabetic patient’s metabolic demand for glucose actually increases. Not only does metabolic activity requiring glucose increase, but patients often fail to consume adequate amounts of food and drink. This leads to a self-fulfilling cycle where the patient feels weak as a result of the infection, fails to eat or drink enough because of said infection that is also increasing glucose demand, and then feels weak once more because of the dropping glucose levels.
Most patients experiencing sepsis will actually develop higher or even hyperglycemic glucose levels, but type 1 diabetic patients may not. As patients fail to compensate for the increased demand, they will eventually develop hypoglycemia. Hypoglycemic symptoms of AMS, weakness, poor oral intake, and tachycardia will only worsen and complicate the pre-existing infection/sepsis issue.
Hypoglycemia episodes in patients experiencing sepsis are prone to higher mortality rates (Mitsuyama et al., 2022).
Alcohol Intoxication
Proteins, lipids, and carbohydrates all contribute to producing glucose when ingested. Alcohol, however, does not. Rather, alcohol has a negative effect on glycogenolysis, glycolysis (the breakdown of glucose), gluconeogenesis (the creation of glycogen), and metabolism overall.
As a result, patients that partake in days-long binge drinking without reprieve or other oral intake may eventually develop hypoglycemia even without any diabetic history. Because of the above stated metabolic effects, patients are unable to restore their glycogen stores or maintain normal blood glucose levels on their own.
Alcohol intoxication can also induce a state of hypoglycemic unawareness. Patients that are intoxicated may not experience the traditional fight or flight effects of catecholamines due to the altered sensory perception and CNS dysfunction that results from alcohol use. If patients are both intoxicated and experiencing hypoglycemic unawareness, they are prone to developing severe hypoglycemia without being able to take their own actions to intervene.
EMS Treatment of Hypoglycemia
For the most part, treatment of hypoglycemia prehospitally is focused around raising blood glucose levels and preventing secondary causes of mortality/morbidity.
Hypoglycemia is one of the most common causes of altered mental status in the United States, and the incidence of diabetes is only increasing (Mulvey, 2023). EMS providers have effective tools to raise blood glucose levels in the field including oral glucose, food, IV dextrose, and glucagon. EMS providers should focus on raising blood glucose in a manner that ensures patient safety while reducing invasive procedures if at all possible.
Many patients with borderline low blood glucose levels will benefit from eating a moderate-sized meal with complex carbohydrates in it. If needed, oral glucose can be provided for a quick boost to blood glucose levels while waiting for the complex carbohydrates to metabolize.
If the patient is altered and can no longer protect their airway/swallow on their own, then IV dextrose is the next line. IV dextrose most commonly comes in D10 (10 percent of the solution is dextrose, so 25 grams are present in 250mL) or D50 (50 percent of the solution is dextrose, so 50mL has 25g of dextrose in it). D10 is preferred as it allows for more precise dosing and is thus less likely to cause rebound hyperglycemia as a part of treatment. D10 is also significantly less necrosive to issue if the IV infiltrates. If the patient is in dire need of glucose administration, intraosseous access can be used.
For every gram of dextrose administered, blood glucose can be expected to increase by 4-6 mg/dL (Fashp, 2022).
Finally, the patient can be provided IM or IN glucagon if IV access is unable to be established. It is important to remember that glucagon only works on the patient’s own pre-existing glycogen stores and will thus produce no response if these stores are depleted.
Works Cited
Cryer, P. E. (2007). Hypoglycemia, functional brain failure, and brain death. Journal of Clinical Investigation, 117(4), 868–870. https://doi.org/10.1172/jci31669
Desimone, M. E. (2018, May 5). Hypoglycemia. Endotext - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK279137/
Dudley, A., Khalil, M. M., Mullins, G., Delanty, N., & Naggar, H. E. (2022). Hypoglycaemic events resembling focal seizures -A case report and literature review. Seizure-european Journal of Epilepsy, 94, 10–17. https://doi.org/10.1016/j.seizure.2021.11.002
Fashp, B. D. H. P. D. F. (2022). EM Pharm Pearls: Estimated rise in blood glucose concentration with dextrose administration. ALiEM. https://www.aliem.com/em-pharm-pearls-estimated-rise-in-blood-glucose-concentration-dextrose/
Hantzidiamantis, P. J. (2022, September 19). Physiology, Glucose. StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK545201/
Khan, M. A., Barlow, R. B., & Weinstock, R. S. (2011). Acute hypoglycemia decreases central retinal function in the human eye. Vision Research, 51(14), 1623–1626. https://doi.org/10.1016/j.visres.2011.05.003
Libretexts. (2023). 14.4B: Adrenergic Neurons and Receptors. Medicine LibreTexts. https://med.libretexts.org/Bookshelves/Anatomy_and_Physiology/Anatomy_and_Physiology_(Boundless)/14%3A_Autonomic_Nervous_System/14.4%3A_Neurotransmitters_and_Receptors/14.4B%3A_Adrenergic_Neurons_and_Receptors
Mitsuyama, Y., Shimizu, K., Komukai, S., Hirayama, A., Takegawa, R., Ebihara, T., Kitamura, T., Ogura, H., & Shimazu, T. (2022). Sepsis‐associated hypoglycemia on admission is associated with increased mortality in intensive care unit patients. Acute Med-Surg, 9(1). https://doi.org/10.1002/ams2.718
Mulvey, A. (2023). More People Being Diagnosed with Type 1 Diabetes. JDRF. https://www.jdrf.org/blog/2020/02/18/more-people-being-diagnosed-type-1-diabetes/#:~:text=A%20new%20report%20from%20the,most%20sharply%20among%20diverse%20populations.