EKGs and What They Mean
Introduction
EKGs are a core part of emergency medicine - after all, average mortality rates from an ST-elevation myocardial infarction can reach up to 24 percent (Vasiljević et al., 2008). In addition to allowing emergency medicine clinicians to identify heart attacks, EKGs allow us to assess a patient’s underlying rhythm for any abnormal findings. EKGs play a critical role in the routine assessment and diagnostics for clinical issues such as syncope, abnormal weakness, chest pain or tightness, or even speech issues.
The goal of this article is not to take a brand new clinician and turn them into an expert in EKG assessment and pathophysiology. Rather, the goal of this article is to provide a solid base of EKG pathophysiology for both novice and intermediate providers.
The Basics - What is an EKG?
An EKG - or electrocardiogram - is a means of assessing the electrical flow through the heart. By assessing the flow of electricity throughout the heart, conditions such as ischemia or myocardial injury can be assessed, localized to a specific area of the heart, and treated.
EKGs are obtained by placing adhesive electrodes on the skin with a cable attached to it. A cardiac monitor attached to the cables is then able to interpret electrical activity as the heart beats.
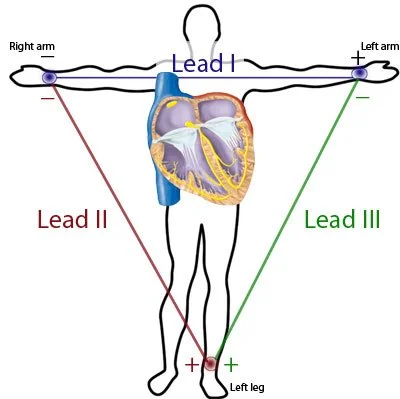
A standard EKG is made up of several “leads” or views of the heart. Each lead is a comparison of two points on the body and the cardiac activity that goes on between them. A basic 3 lead EKG (as in, there are 3 lead present for assessment) is based off the comparison of 3 different positive (or interpreting leads) and 1 grounding lead. This principle is the called the Einthoven’s Triangle. See below for a graphical representation of this concept.
Lead I - Lead I is a comparison of electrical flow between the left and right arms. Any flow of energy running from the right side of the body towards the left arm will produce waveforms in lead I.
Lead II - Lead II is a comparison of electrical flow between the left leg (positive lead) and right arm.
Lead III - Lead III is a comparison of electrical flow, as shown above, between the left leg and left arm.
The right leg serves as the grounding electrode to allow for accurate assessment of the other leads. It does not collect diagnostic information on its own. Because of this, this is why this method of EKG assessment is a called 3 lead - as only 3 leads are actually being captured.
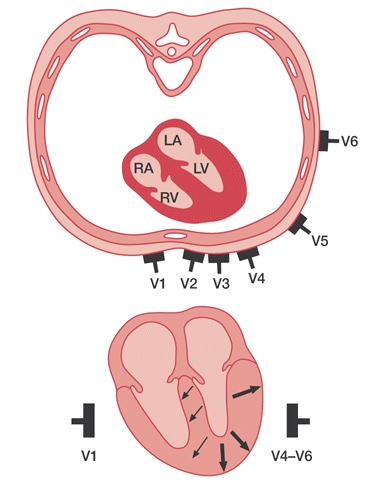
A 12 lead adds on the precordial leads - aka the chest leads. These leads are labeled V1-V6 and allow for a more in-depth localization of the heart. This is used to identify myocardial ischemia throughout the heart depending on the coronary artery vessels involved. With each coronary vessel feeding a different section of the heart, clinicians are able to identify which vessel is involved.
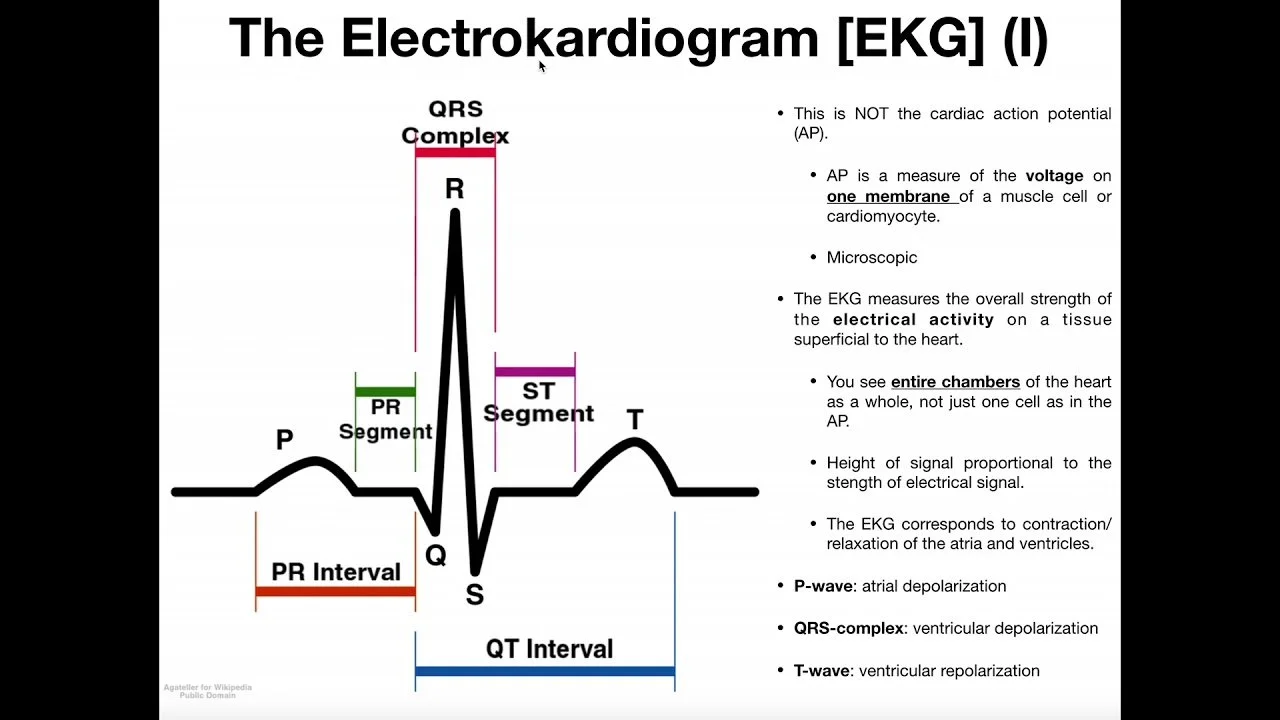
The Parts of an EKG
Specific Rhythms
The main purpose of this article is to explore the pathophysiology behind each common rhythm. By understanding the pathophysiology behind each rhythm, it is easier to anticipate rhythm changes and the treatment required as an EMS provider.
Why do rhythms change?
Rhythms change for a variety of reasons, but it’s most often due to either an erratic, unstable section of the heart taking over pacemaking or as an escape mechanism to compensate for failure of higher sections of the heart during times of myocardial stress.
It is important to note that each and every section of the heart has an instrinsic rate - that is, the normal pacemaking rate that it will perform. We will cover each instrinsic rate throughout this article.
All pacemaking in the heart initially begins at the level of the sinus node. This is a section of pacemaking cardiac cells located in the right atrium of the heart. The SA node is responsible for all normal pacemaking in the heart, and can usually have a healthy heart rate up to 220 - patient’s age. The sinus node can produce normal sinus rhythm (sinus-originating rhythm between 60-100, aka the intrinsic rate), sinus bradycardia (sinus-originating rhythm under 60 BPM), and sinus tachycardia (sinus-originating rhythm over 100 BPM). Rhythms originating from the sinus node are narrow complex (under 120ms), organized, and have distinct, regular P waves before each QRS complex. While it is possible for ectopic areas of the heart to specifically interfere with P waves (such as premature atrial contractions), it is not the goal of this article to discuss that in full detail.
In times of myocardial ischemia, such as during a myocardial infarction or simply global hypoxia/hypoperfusion, the SA node can fail and force the heart to rely on other, lower pacemaking cells to maintain a heart rhythm. For instance, a blockage of the right coronary artery - which typically feeds both the SA node and the AV junction - can cause such profound ischemia that the SA node fails and the ventricles have to take over pacemaking responsibilities for the heart.
The atria themselves can also service as an ectopic area of the heart, resulting in atrial fibrillation, atrial flutter, and atrial tachycardia. Atrial rhythms are usually prompted by either secondary factors such as disease or CHF or from structural issues such as congenital AFib. Another article on this site will discuss atrial rhythms in further detail and will be linked here when it is completed.
Electrical conduction then passes from the SA node down through the atrioventricular node, which is found in the upper septum of the heart between the atria. The purpose of the AV node is to connect the SA node with the rest of the heart and allow for continuous conduction. The intrinsic rate of the AV node is 40-60 BPM, and it can produce a junctional escape rhythm (40-60 BPM), accelerated junctional escape rhythm (60-100 BPM) or junctional tachycardia (junctional-originating rhythm over 100 BPM). If the SA node fails, the AV node is the next in line to take on normal pacemaking.
Electrical conduction then passes through the Bundle of His and down into the ventricles. Keep in mind that the Bundle of His is also capable of ectopic pacemaking and can produce a junctional rhythm (Hafeez, 2023).
These sections above are responsible for a collection of rhythms known as supraventricular tachycardia. This is essentially any section of the heart above the ventricles (as the name implies) prompting a heart rhythm with an BPM over 150. Supraventricular tachycardia (SVT) is not a rhythm in and of itself, but rather a descriptor for a collection of rhythms that exist above the ventricles.
Some basic notes for rhythm interpretation is that anything that is causing increasing aberrant conduction or working primarily through the ventricles of the heart is going to show a widened (over 120ms) QRS on the monitor.
The ventricles will then take over pacemaking responsibility if the upper sections of the heart fail.
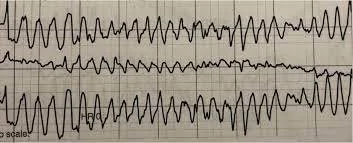
Torsades de Pointes
Torsades de pointes, also known as “twisting of the points,” is an erratic and polymorphic form of ventricular tachycardia. It typically begins with a gradual prolongation of the QRS complexes that turns into ventricular tachycardia and then into torsades de pointes. It is mostly strong associated with conditions of QTc prolongation (Cohagan, 2022) and can occur after an R on T occurrence. Medications or congenital heart issues can prompt QT prolongation. One common EMS medication that has the potential to induce QT prolongation is Ondansetron (Zofran), for instance.
Patients can remain awake and alert while experiencing torsades de pointes - it is a not a classic cardiac arrest rhythm.
Remember - ventricular-originating rhythms are wide complex, as all other pacemaking areas of the heart have failed or been overtaken by the ventricles. This is why the QRS is wide (over 120ms duration).
First line treatment for this rhythm is magnesium sulfate administration via IV. Magnesium sulfate works by halting the influx of calcium ions into the cardiac myocytes, and thus halting the erratic muscular contraction of the ventricles (Munro & Graham, 2002). Remember - calcium and sodium are the cations responsible for part of the sodium-potassium ion pump. On the other hand, potassium is the major anion that is responsible for prompting repolarization. The influx of sodium and calcium shifts the polarity of the outer plasma membrane to highly positive, prompting a contraction. If you are able to provide magnesium, which counteracts the action of calcium, you are able to stop the constant and erratic depolarizations and thus restore the heart to a normal rhythm.
Torsades de pointes is likely to progress to a full cardiac arrest if it is unstable and remains untreated.
Digoxin Effect (12 Lead)
This is a finding on 12leads characterized by ST segment “sagging” in addition to biphasic T waves and a short QT interval. It also frequently presents with a U shape in place of the T wave. This is the result of digoxin, a common heart failure medication, use. It is often called the “Salvador Dali” effect as the actual morphology appears similar to the famous painter Salvador Dali’s mustache.
The specific features of this 12lead sign are due to the shortening effects that digoxin has on the cardiac refractory period (LITFL).
What is Digoxin?
Digoxin is a cardiac glycoside medication used to increase contractility (positive inotrophy effect) and slow electrical conduction through the AV node. As a result, the heart pumps stronger but less - which is ideal for a situation in which you’re managing congestive heart failure. Digoxin specifically works by increasing sodium influx into the heart via the sodium-potassium pump and thus causing stronger heart contractions (David, 2023). With the goal of CHF management being to reduce both preload and afterload of the heart, this is beneficial and allows the heart to clear more fluid - thus reducing preload specifically.
This article is a work in progress designed to become a long-read, easily accessible guide to EKG patterns and their pathophysiologies.
Last update: 6/16/2023 at 0000.